When a molecule absorbs light, it undergoes a whirlwind of quantum-mechanical transformations. Electrons jump between energy levels, atoms vibrate, and chemical bonds shift — all within millionths of a billionth of a second.
These…
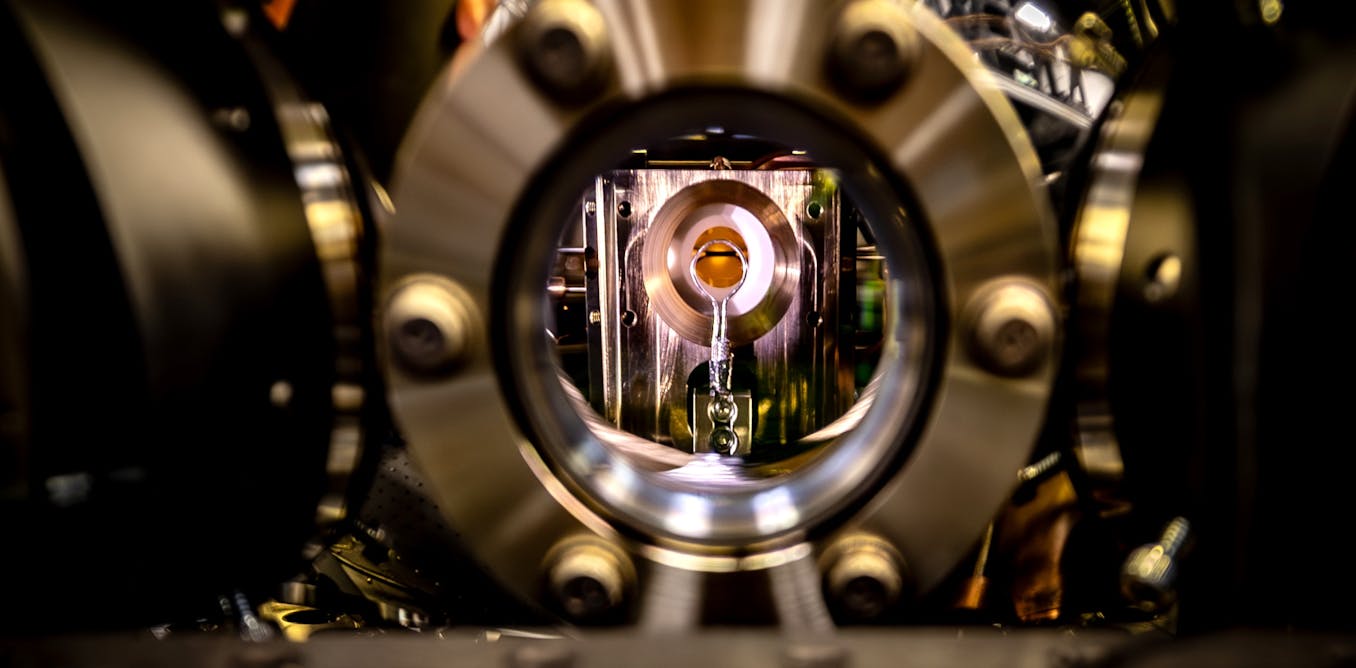
When a molecule absorbs light, it undergoes a whirlwind of quantum-mechanical transformations. Electrons jump between energy levels, atoms vibrate, and chemical bonds shift — all within millionths of a billionth of a second.
These…