Study design and participants
This phase 2 single-arm, open-label trial (ClinicalTrials.gov NCT05348213) adopted a prospective cohort design. Enrollment was restricted to R/R DLBCL patients. Participant inclusion criteria included: age 18–75…
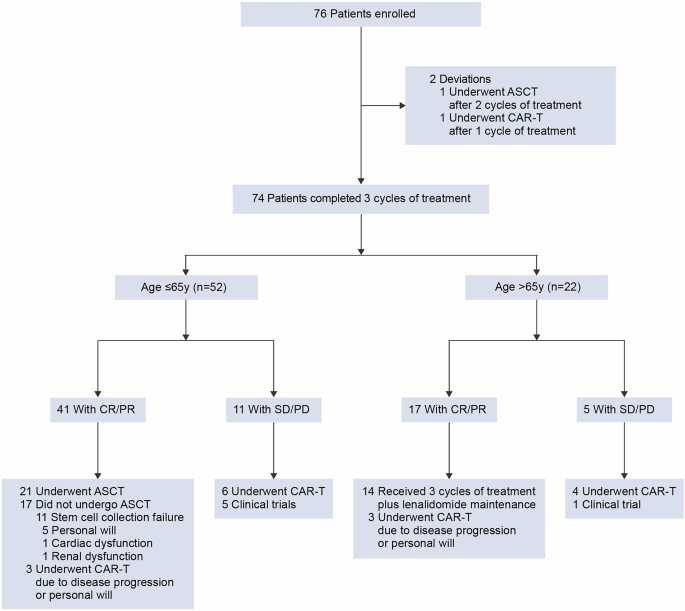
This phase 2 single-arm, open-label trial (ClinicalTrials.gov NCT05348213) adopted a prospective cohort design. Enrollment was restricted to R/R DLBCL patients. Participant inclusion criteria included: age 18–75…